FEATURE
Impact of Colloidal Silica Loading on Adhesive Properties and Film Morphology of High-Performance Waterborne Acrylic Pressure-Sensitive Adhesives
Part 1: Introduction and Experimental
Impact of Colloidal Silica Loading on Adhesive Properties and Film Morphology of High-Performance Waterborne Acrylic Pressure-Sensitive Adhesives
Part 1: Introduction and Experimental
By Kylie M. Kennedy, Ph.D., Research Scientist, Acrylic Adhesives; Joseph B. Binder, Ph.D., Senior Research Scientist, Acrylic Adhesives; Edward L. Lee, Ph.D., Research Scientist, Acrylic Adhesives; Johnpeter Ngunjiri, Ph.D., Research Scientist, Core R&D Analytical Sciences; Michael A. Mallozzi, R&D Technician Leader, Acrylic Adhesives; William DenBleyker, R&D Technician, Acrylic Adhesives, The Dow Chemical Co., Collegeville, Pennsylvania
This paper was honored with the 2024 Carl Dahlquist Award at the Pressure Sensitive Tape Council’s Tape Week 2024.
Abstract
Many high-performance pressure-sensitive adhesive (PSA) applications are dominated by solventborne and radiation-curable PSAs because they can achieve greater cohesive strength and heat resistance than waterborne technologies. However, waterborne emulsion PSAs provide many advantages such as high solids, low cost, safe handling, and environmental benefits. With these advantages in mind, industry and academia alike have sought to close performance gaps between waterborne emulsion PSAs and other technologies, especially by achieving high adhesion in combination with high shear and high-temperature resistance.
Inorganic nanofillers have been incorporated in situ during polymerization or as post-polymerization additives to improve the mechanical properties of waterborne acrylic adhesives. The method of introduction and the resulting filler-filler interactions and networks within the dried film are extremely important to the final mechanical properties. In this study, colloidal silica (Levasil CT14 PNM) (CS) and an experimental nanoparticle (EN) filler were added to a bimodal particle size, high solids (>60% total solids), poly(butyl acrylate)-co-(methacrylic acid) waterborne PSA emulsion at 0, 2, 4, 6, and 8 wt% on a solids on total weight of latex basis. These formulations were coated on 2-mil PET to prepare laminates under industrially relevant drying conditions at two different coating weights. PSA performance (peel adhesion, loop tack, shear resistance, and shear adhesion failure temperature (SAFT)) were assessed on both high-density polyethylene (HDPE) and stainless steel (SS) substrates. Notably, CS addition improved the shear resistance while maintaining peel adhesion. Furthermore, the SAFT increased from 100°C to over 170°C with CS addition. The EN additive also improved shear resistance and SAFT, while maintaining adhesion. Interestingly, loop tack was also improved on both HDPE and SS substrates at higher coating weight. Atomic force microscopy (AFM) of the surface of the dried films was used to probe how the morphology may relate to PSA performance.
Introduction
A common challenge when designing polymers for pressure-sensitive adhesive (PSA) applications is the inverse relationship between cohesion (shear strength) and adhesion (peel and tack) properties. High shear resistance can be obtained by increasing the PSA stiffness, but higher modulus can lead to poor and limited fibrillation during debonding. Viscous behavior is required for high peel and tack, but this typically reduces shear strength.1
Finding ways to improve adhesion/cohesion balance and improving high-performance properties of waterborne PSAs is particularly desirable. High-performance PSAs typically rely on the use of solventborne or UV-syrup technologies owing to their inherently homogenous films that often result in high shear resistance and temperature resistance.2 With increasing environmental concerns and consumer safety awareness, liquid formulations are required to be more sustainable with one strategy being the use of water as the dispersing medium. The elimination of organic solvent limits material waste and reduces the amount of volatile organic compounds (VOC)s in the product, improving environmental impact and consumer safety. Waterborne PSAs, predominately waterborne colloidal dispersions, offer advantages such as high solids, low cost, safe handling, and environmental benefits. The challenge however is that drying of waterborne emulsion PSAs result in inherently heterogeneous films during water evaporation, which tends to negatively impact high-performance characteristics.3–6 More sustainable waterborne PSA product offerings would ideally come without any performance gaps at comparable or lower cost compared to solventborne counterparts.
Although performance requirements will vary based on the PSA application, balancing peel resistance (a fast deformation process) and resistance to shear (a slow deformation process) is generally required but these properties are difficult to independently tune.7 The PSA must dissipate energy during peeling (typical of viscous liquids) and be resistance to creep under shear (characteristic of crosslinked materials).7 Increasing network density via crosslinking is a traditional method of PSA reinforcement in both solventborne and waterborne adhesives.2,8,9 However, void spaces in latex PSAs are not typically addressed by crosslinking. Crosslinking also reduces the viscous properties of the polymer network, reducing the ability of the polymer to flow into the substrate, resulting in reduced adhesion.10,11 Furthermore, from a polymerization standpoint, factors such as monomer composition, sol, and gel parameters are difficult to independently control by free radical polymerization, which is the typical method for producing waterborne acrylic PSAs.1,7,12,13
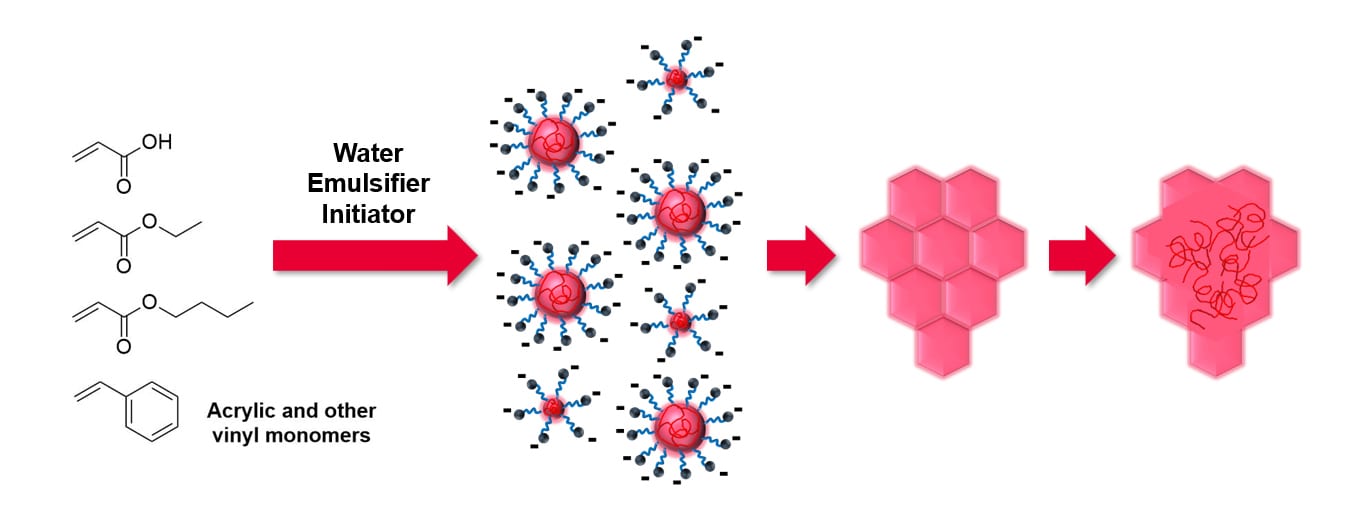
As previously mentioned, in addition to synthetic limitations, waterborne latex emulsions form inherently more heterogeneous films. Polymers typically used in waterborne PSAs typically have a low glass transition temperature (Tg) with latex particles on the order of 50 nm to 1 µm (Figure 1.). Once the latex dispersion is cast on a substrate and water starts evaporating, the polymer chains have sufficient mobility to diffuse across polymer particles and ideally form a cohesive film. Typically, the major components of a PSA dispersion are a hydrophobic copolymer of acrylic, styrenic, or other vinyl monomers made by emulsion polymerization with surfactants and ionized or polar side chains surrounding the hydrophobic particles. In dried films, hydrophilic components can disrupt uniform particle coalescence, forming weak boundary layers between particles, and ultimately this can result in compromising PSA performance. Water-soluble polymers have been used to endow a percolating network that serves as a bridge between latex particles that do not fully coalesce, improving shear resistance.14–16 This improvement in shear resistance can however be met with a reduction in peel adhesion due to special confinement of the elastomeric latex phase.17
Using a nanocomposite strategy is one way to impart mechanical strength to the interfaces between latex particles and can result in PSAs with balanced cohesion and adhesion properties. Inorganic nanofillers such as carbon nanotubes (CNTs),18,19 silica fillers,6,20 and hard nanoparticles21 are often incorporated in aqueous polymer dispersions to obtain colloidal composite mixtures with enhanced properties. The distribution of the nanoparticles during film drying is highly relevant to the mechanical, thermal, and chemical resistance properties of the resulting PSA. Several preparation methods, such as Ramsden-Pickering emulsions and in situ polymerization have been used to make aqueous-stable nanocomposites mixtures.22–28 The main objective of this approach is that in the dried film the inorganic fillers are homogenously distributed with the filler often acting as a stabilizer at the surface of the polymer colloids. Pickering emulsions using inorganic nanoparticles can partly hinder the diffusion of polymer chains between particles, consequentially increasing the film formation temperature.29,30 On the other hand, in situ polymerization can initiate a discontinuous or aggregated inorganic phase in the resulting nanocomposite. The mechanical properties of the films are extremely dependent on effective filler-filler interactions and the networks eventually formed.31,32 Coalescence via molecular diffusion of polymer chains throughout particle boundaries is again key to targeting a homogeneous solid film in both the Pickering emulsion and in-situ polymerization routes.33
Straightforward mixing of pre-prepared colloids of different types in an aqueous colloidal dispersion is advantageous in that the polymer-filler weight ratio can be easily varied. Creating a stable colloidal dispersion in the case of mixing pre-made colloids is often mediated by the presence of surfactants or stabilizers. The presence of free-moving stabilizers and surfactants often play a key role not only in the stability of the dispersion, but also strongly influence the assembly processes occurring in the aqueous state and resulting morphology of the final dried film. Mixing different colloidal types can also result in phenomenon such as aggregation, deposition, and stratification.34,35 For example, colloids of different sizes have different mobility in the wet/drying film, and particles with a large Peclet number tend to be trapped at the air-coating interface.36 In general, it has been reported that the presence of a 3D percolating network of both filler and polymer phases is a prerequisite for good performance of waterborne coatings with improved mechanical properties.35
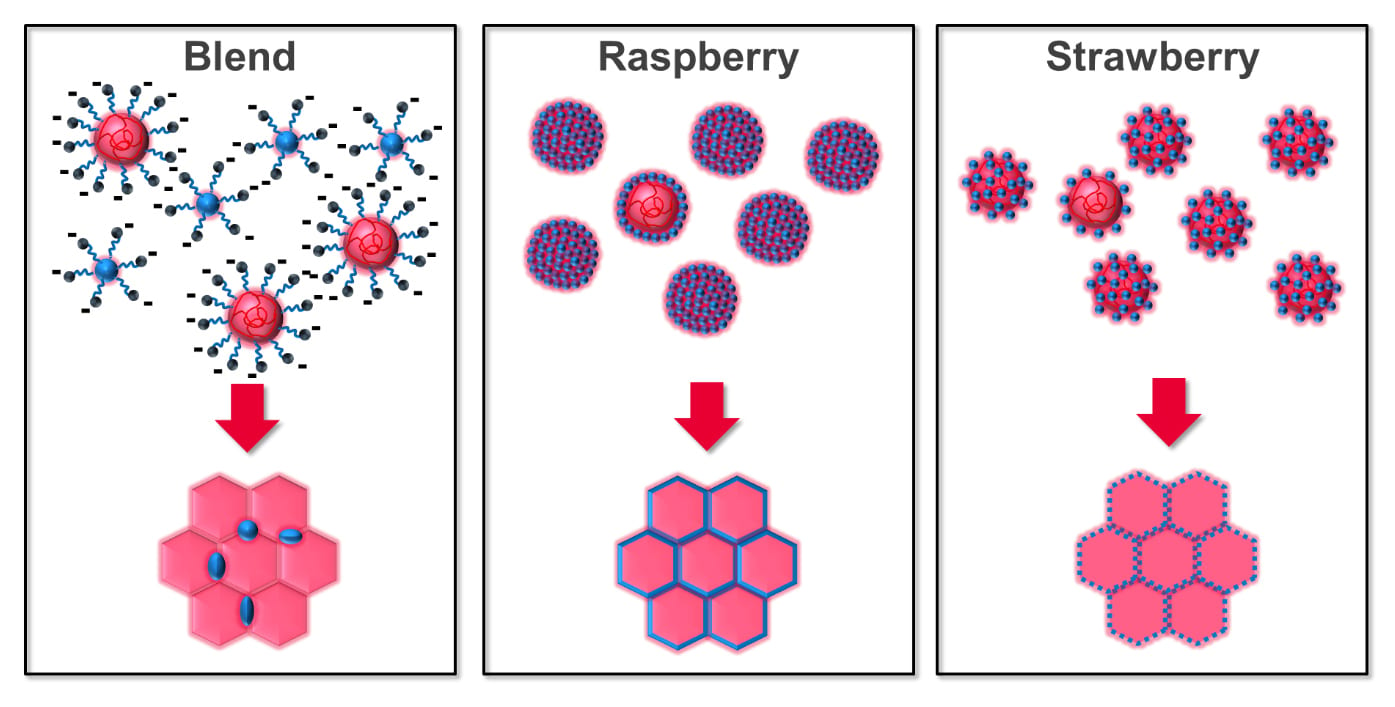
The methods above have been used to prepare waterborne polymer-silica colloidal systems22–28,37,38 for coatings with improved mechanical and thermal performance. Typically, a core-corona morphology is obtained using particles of varying sizes and ratios (Figure 2). Most relevant, the core consists of a spherical latex particle, and the corona is a layer of silica nanoparticles (typically spherical) in either a raspberry24,25 (i.e. polymer core fully covered by corona layer) or strawberry (i.e. partial corona coverage of core latex particle).34,35 Pickering nanocomposite dispersions often lead to the formation of raspberry configurations where molecular diffusion can be hindered between particle boundaries33 often resulting in a higher film formation temperature29,30 and reduced optical clarity or reduced mechanical properties.24,25,39 Strawberry configuration tends to generate films with better film formation properties (i.e. reasonable molecular diffusion of polymer chains through particle boundaries, low aggregation of nano-fillers, and continuous 3D nanonetworks).34 Using functionalized forms of colloidal silica and polymer grafted nanoparticles are also methods to improve compatibility of the latex and silica nanofillers on film drying.35,40
Figure 1. Waterborne PSAs can be tuned at multiple scales, including selection of monomers, design of particles, and coalescence into films.
The use of nanoparticle additives in commodity industrial applications can sometimes be cost-prohibitive and these additives often need to be used in appreciable amounts for performance enhancement. Using additives with particularly small size, but high surface area, can improve the efficacy of a nanofiller approach. In the present study, colloidal silica (CS) was added to a bimodal particle size waterborne emulsion PSA from 0-8% solid nanoparticle on total weight of wet latex. Use of bimodal particle sizes allows production of a latex with high solids content (>60% total polymer solids). Adding colloidal silica to a waterborne PSA is not novel,41 but we are not aware of any prior reports where the CS is added to a bimodal waterborne PSA. Understanding how CS nanofiller loading impacts performance in a high-solid, bimodal particle size system is more industrially relevant. Furthermore, the laminates in the present study were dried using industrially relevant drying conditions and coating methods.
We have investigated the impact of mixing spherical inorganic CS and an experimental nanoparticle (EN) with a high solid waterborne acrylic latex on PSA performance. Films were cast and dried using industrially relevant conditions, and peel resistance, shear resistance, and loop tack were measured on high-density polyethylene (HDPE) and stainless-steel (SS) substrates. The shear adhesion failure temperature (SAFT) was also measured to probe high-temperature performance of the PSAs. Atomic force microscopy (AFM) was used to study how the morphology of the film may relate to PSA performance.
Experimental Materials
Levasil CT14 PNM was provided by Nouryon and used with no modifications and are referred to as colloidal silica (CS). Experimental nanoparticles (EN) were prepared by a proprietary method.
Preparation of Pressure Sensitive Adhesive
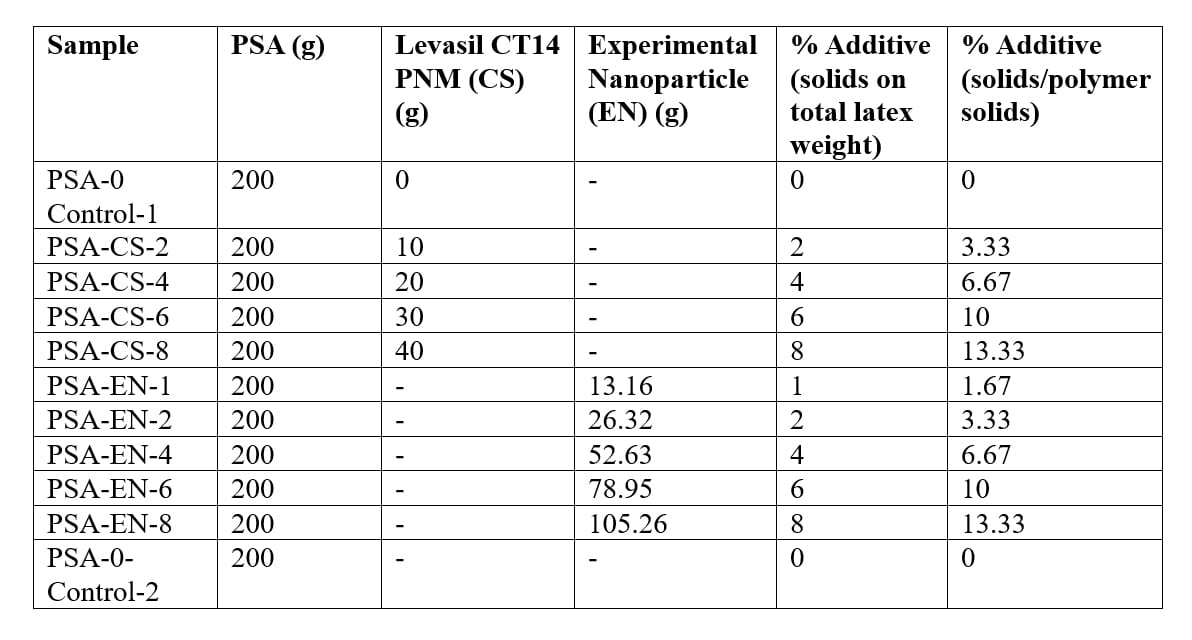
The pressure sensitive adhesive dispersion was prepared by a typical semi-continuous emulsion polymerization process using persulfate initiator and monomer composition by weight of 98.2 butyl acrylate (BA)/1.8 methacrylic acid (MAA). A bimodal particle size distribution was targeted for the preparation of a high polymer solids binder (>60% total polymer solids). The pH of the binder was adjusted to 9.5 with aqueous ammonia to prevent coagulation upon the additional of high pH colloidal silica. The control latex polymers (PSA-0-Control-1 and PSA-0-Control-2) differ only by one post-additive surfactant.
PSA Dispersion-Nanoparticle Blend Preparation
The nanoparticle additives CS and EN were added dropwise to wet latex (200 g) with overhead mechanical stirring. Each blend was then stirred for an additional 10 minutes after the adding the nanoparticle additive. The additives were added at 0–8% actives on total latex weight.
Figure 2. Effects of latex particle morphology on film structure including blend with a nanofiller, raspberry particles, and strawberry particles.
Latex/Blend Characterization
Total Solids
Total solids are determined by weighing latex (about 1 g) on a tared aluminum pan, baking it at 150 ˚C for 30 minutes, and re-weighing the sample. Alternatively, latex (0.5 g) may be weighed into the pan of moisture balance for total solids determination.
pH
Latex pH is measured using an Apera Instruments Model pH8500 meter calibrated with pH 4, 7, and 10 buffer solutions.
Viscosity
Latex viscosity is measured using a Brookfield LV viscometer with spindle #3 at 30 rpm after bringing the latex sample to 25 °C using a water bath.
Application Testing Procedures
All PSTC methods are from the Pressure Sensitive Tape Council (Chicago, Illinois).
Laminate Preparation from PSA Dispersion-Colloidal Silica Nanoparticle Blend Preparation
Adhesive laminates were prepared by direct coating onto 2 mil polyethylene terephthalate (PET) film using a 3 mil (0.003”) depth stainless steel bird bar for target dry coat weight of 50 gsm or 0.8 mil (0.0008”) depth bird bar for 18 gsm and closed with a siliconized release liner (RP-12). The wet drawdowns were dried in a convection oven at 80 °C for 15 mins and 5 mins, respectively. The laminates were then passed slowly (100 inches/min) through a heated laminator at 40 °C/40 psi and finally conditioned in a controlled temperature room (CTR, 23 °C, 50 % humidity) overnight prior to applications testing.
Peel Adhesion (PSTC Test Method 101)
Peel adhesion is the measure of the adhesive strength of the adhesive to the substrate. The peel adhesion is tested by applying a 1” wide strip to a chosen substrate (typically stainless steel or high-density polyethylene (HDPE) panels) using the ChemInstruments roll-down machine (Model RD-1000/3000, 4.5 lb roller, moving at 24 in/min, rolled twice in each direction). The adhesive is allowed to dwell on the panel for 1 min (first half of panel) and 24 hr (second half of same panel) and then peeled from the panel at a 180° angle at 12 in/min using an Instron tensile tester. The average max force and standard deviation of 4 replicates are reported along with the failure modes.
Loop Tack (PSTC Test Method 16A)
The Loop Tack test measures the initial adhesion when the adhesive comes in contact with the substrate. A 1” wide x 7” long strip is cut and folded over to form a 5” loop, exposing the adhesive side. It is then placed in between the jaws of the Instron and lowered at a rate of 12 in/min to the substrate (HDPE or stainless steel) such that a 1” x 1” area of the adhesive comes in contact with a pristine area of the substrate for 10-15 seconds. Then the adhesive is pulled away and the peak force to pull the adhesive away from the substrate is recorded from 3 replicates along with the failure mode.
Shear (PSTC Test Method 107)
Shear is a measure of the cohesive strength of the adhesive. A 1” x 1” label sample is applied to a stainless-steel panel and laminated using a roll-down machine (4.5 lb roller, moving at 24 in/min, rolled twice in each direction) and allowed to dwell for 5 minutes. The panels are mounted vertically on a shear bank at an angle of 2°. A 1 kg weight is hung at the bottom of the sample. The time to failure is recorded as the shear (in hours) along with the failure modes. Typically, the failure mode is cohesive, thus directly correlated with the internal strength of the adhesive. The average of 3 replicates are reported.
SAFT (PSTC Test Method 17)
The sample strips were also tested for SAFT (Shear Adhesion Failure Temperature) using the method PSTC-17. A 1” x 1” label sample is applied to a stainless-steel (SS) panel and laminated using a roll-down machine (4.5 lb roller, moving at 24 in/min, rolled twice in each direction) and allowed to dwell for 5 minutes. The steel panel with the strip is held in a rack in a 40 °C oven (Gruenberg oven equipped with a Yokogawa programmable controller) such that the panel forms an angle of 178 degrees to 180 degrees. Then the 1-kg weight is hung on the strips. The oven is programmed to hold at 40 °C for 20 minutes immediately after the weight is hung. After the 20-minutes hold, the oven temperature increases at a rate of 0.5 °C per minute. When the oven temperature reaches 205 °C, the test is completed, and the oven starts to cool. When the 1-kg weight drops due to the failure of test strips on steel panel, the temperature is recorded as SAFT. If test strip does not fail throughout the course of temperature ramp, the SAFT is recorded as 205+°C. The average of 3 replicates are reported along with the failure mode.
Removability Testing
A 1” wide sample adhesive stripwas applied to aSS panel, pressed down using a roll-down machine (4.5 lb roller, moving at 24 in/min, rolled twice in each direction), and allowed to dwell for 5 minutes. The samples were placed in a convective oven at 120 °C for 20 mins or 60 mins, cooled to room temperature, and conditioning in a CTR for 20 mins. The peel force was measured following the 180° peel adhesion test.
Failure Modes
Failure modes are abbreviated as follows:
- AF: Adhesive failure
- CF: Cohesive failure
- AFB: Adhesive Failure from Backing
- If a mixture of different failure modes were observed among the 4 replicates for a given sample, they are marked as mixed failures, such as mix AF/AFB. Occasionally, the abbreviation “sl” is listed to indicate a slight failure mode.
Atomic Force Microscopy (AFM) Imaging of PSA Films
Adhesive laminates were prepared by direct coating onto 2 mil polyethylene terephthalate (PET) film using a 3 mil (0.003”) depth stainless steel bird bar for target dry coat weight of 50 gsm and closed with a siliconized release liner (RP-12). The wet drawdowns were dried in a convection oven at 80 °C for 15 mins and 5 mins, respectively. The laminates were then passed slowly (100 inches/min) through a heated laminator at 40 °C/40 psi and finally conditioned in the CTR (23 °C, 50 % humidity) overnight prior to applications testing. Freshly peeled PSA coatings on PET samples were analyzed in Tapping mode AFM using a Bruker Dimension Icon AFM (Bruker, Santa Barbara, CA). Tapping Mode is an AFM imaging technique that lightly taps the surface with an oscillating probe tip. During imaging, the cantilever’s oscillation amplitude is maintained constant using a z feedback loop. As the tip rasters on the surface, changes in sample surface topography impact the cantilever’s oscillation amplitude. Phase signal (delay in oscillation) is sensitive to variations in material composition by highlighting properties such as adhesion, friction, viscoelasticity, and contact area.
Calculation of Surface Roughness (Rq) by AFM
Coating roughness (Rq) was calculated from 10 um2 images to capture both CS and EN particle contribution and the acrylic particle shapes at the surface.
Table 1. Formulations of PSA with Nanoparticle Dispersions (by weight).
Acknowledgements
The authors would like to thank Dow Adhesives and Core R&D Analytical Sciences for their support of this work as well as PSTC for the opportunity to present it.
References
1. Tobing, S., Klein, A., Sperling, L. H. & Petrasko, B. Effect of network morphology on adhesive performance in emulsion blends of acrylic pressure sensitive adhesives. J. Appl. Polym. Sci. 81, 2109–2117 (2001).
2. Czech, Z. & Milker, R. Development trends in pressure-sensitive adhesive systems. 2005 23, 0137–1339.
3. Mallégol, J., Bennett, G., McDonald, P. J., Keddie, J. L. & Dupont, O. Skin Development during the Film Formation of Waterborne Acrylic Pressure-Sensitive Adhesives Containing Tackifying Resin. J. Adhes. 82, 217–238 (2006).
4. Felton, L. A. Mechanisms of polymeric film formation. Int. J. Pharm. 457, 423–427 (2013).
5. Nollenberger, K. & Albers, J. Poly(meth)acrylate-based coatings. Int. J. Pharm. 457, 461–469 (2013).
6. Ribeiro, T., Baleizão, C. & Farinha, J. Functional Films from Silica/Polymer Nanoparticles. Materials 7, 3881–3900 (2014).
7. Bellamine, A. et al. Design of Nanostructured Waterborne Adhesives with Improved Shear Resistance. Macromol. Mater. Eng. 296, 31–41 (2011).
8. Yang, J. et al. The effect of molecular composition and crosslinking on adhesion of a bio-inspired adhesive. Polym. Chem. 6, 3121–3130 (2015).
9. Wahdat, H., Gerst, M., Rückel, M., Möbius, S. & Adams, J. Influence of Delayed, Ionic Polymer Cross-Linking on Film Formation Kinetics of Waterborne Adhesives. Macromolecules 52, 271–280 (2019).
10. Lee, J.-H., Shim, G.-S., Kim, H.-J. & Kim, Y. Adhesion Performance and Recovery of Acrylic PSA with Acrylic Elastomer (AE) Blends via Thermal Crosslinking for Application in Flexible Displays. Polymers11, 1959 (2019).
11. Czech, Z. Synthesis and cross-linking of acrylic PSA systems. J. Adhes. Sci. Technol. 21, 625–635 (2007).
12. Tobing, S. D. & Klein, A. Molecular parameters and their relation to the adhesive performance of acrylic pressure‐sensitive adhesives. J. Appl. Polym. Sci. 79, 2230–2244 (2001).
13. Tobing, S. D. & Klein, A. Molecular parameters and their relation to the adhesive performance of emulsion acrylic pressure‐sensitive adhesives. II. Effect of crosslinking. J. Appl. Polym. Sci. 79, 2558–2564 (2001).
14. Gurney, R. S. et al. Mechanical properties of a waterborne pressure-sensitive adhesive with a percolating poly(acrylic acid)-based diblock copolymer network: Effect of pH. J. Colloid Interface Sci. 448, 8–16 (2015).
15. Peruzzo, P. J. et al. On the strategies for incorporating nanosilica aqueous dispersion in the synthesis of waterborne polyurethane/silica nanocomposites: Effects on morphology and properties. Mater. Today Commun. 6, 81–91 (2016).
16. Kennedy, K. M. et al. Variation in adhesion properties and film morphologies of waterborne pressure‐sensitive adhesives containing an acid‐rich diblock copolymer additive. J. Appl. Polym. Sci. 140, (2023).
17. Deplace, F. et al. Deformation and adhesion of a periodic soft–soft nanocomposite designed with structured polymer colloid particles. Soft Matter 5, 1440–1447 (2009).
18. Wang, T. et al. Waterborne, Nanocomposite Pressure‐Sensitive Adhesives with High Tack Energy, Optical Transparency, and Electrical Conductivity. Adv. Mater. 18, 2730–2734 (2006).
19. Yang, J., Zhang, Z., Friedrich, K. & Schlarb, A. K. Creep Resistant Polymer Nanocomposites Reinforced with Multiwalled Carbon Nanotubes. Macromol. Rapid Commun. 28, 955–961 (2007).
20. Wang, Z. D. & Zhao, X. X. Creep resistance of PI/SiO2 hybrid thin films under constant and fatigue loading. Compos. Part A: Appl. Sci. Manuf. 39, 439–447 (2008).
21. Zhang, Z., Yang, J.-L. & Friedrich, K. Creep resistant polymeric nanocomposites. Polymer 45, 3481–3485 (2004).
22. Novak, B. M. Hybrid Nanocomposite Materials—between inorganic glasses and organic polymers. Adv. Mater. 5, 422–433 (1993).
23. Zou, H., Wu, S. & Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 108, 3893–3957 (2008).
24. Percy, M. J. & Armes, S. P. Surfactant-Free Synthesis of Colloidal Poly(methyl methacrylate)/Silica Nanocomposites in the Absence of Auxiliary Comonomers. Langmuir 18, 4562–4565 (2002).
25. Tiarks, F., Landfester, K. & Antonietti, M. Silica Nanoparticles as Surfactants and Fillers for Latexes Made by Miniemulsion Polymerization. Langmuir 17, 5775–5780 (2001).
26. Bourgeat-Lami, E. & Lang, J. Encapsulation of Inorganic Particles by Dispersion Polymerization in Polar Media 1. Silica Nanoparticles Encapsulated by Polystyrene. J. Colloid Interface Sci. 197, 293–308 (1998).
27. Schoth, A., Landfester, K. & Muñoz-Espí, R. Surfactant-Free Polyurethane Nanocapsules via Inverse Pickering Miniemulsion. Langmuir 31, 3784–3788 (2015).
28. Schmid, A., Tonnar, J. & Armes, S. P. A New Highly Efficient Route to Polymer‐Silica Colloidal Nanocomposite Particles. Adv. Mater. 20, 3331–3336 (2008).
29. González-Matheus, K., Leal, G. P. & Asua, J. M. Film formation from Pickering stabilized waterborne polymer dispersions. Polymer 69, 73–82 (2015).
30. Negrete‐Herrera, N., Putaux, J., David, L., Haas, F. D. & Bourgeat‐Lami, E. Polymer/Laponite Composite Latexes: Particle Morphology, Film Microstructure, and Properties. Macromol. Rapid Commun. 28, 1567–1573 (2007).
31. Oberdisse, J., Hine, P. & Pyckhout-Hintzen, W. Structure of interacting aggregates of silica nanoparticles in a polymer matrix: small-angle scattering and reverse Monte Carlo simulations. Soft Matter 3, 476–485 (2006).
32. Kosugi, K., Arai, H., Zhou, Y. & Kawahara, S. Formation of organic–inorganic nanomatrix structure with nanosilica networks and its effect on properties of rubber. Polymer 102, 106–111 (2016).
33. Keddie, J. L. & Routh, A. F. Fundamentals of Latex Film Formation, Processes and Properties. Springer Lab. 151–183 (2010) doi:10.1007/978-90-481-2845-7_5.
34. Li, S. et al. Assembly of partially covered strawberry supracolloids in dilute and concentrate aqueous dispersions. J. Colloid Interface Sci. 627, 827–837 (2022).
35. Li, S., Ven, L. G. J. van der, Spoelstra, A. B., Tuinier, R. & Esteves, A. C. C. Tunable distribution of silica nanoparticles in water-borne coatings via strawberry supracolloidal dispersions. J. Colloid Interface Sci. 646, 185–197 (2023).
36. Fortini, A. et al. Dynamic Stratification in Drying Films of Colloidal Mixtures. Phys. Rev. Lett. 116, 118301 (2016).
37. Bleier, A. & Matijević, E. Heterocoagulation. Part 3.—Interactions of polyvinyl chloride latex with Ludox Hs silica. J. Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases74, 1346–1359 (1978).
38. Balmer, J. A. et al. Unexpected Facile Redistribution of Adsorbed Silica Nanoparticles Between Latexes. J. Am. Chem. Soc. 132, 2166–2168 (2010).
39. Scherer, G. W. Theory of Drying. J. Am. Ceram. Soc. 73, 3–14 (1990).
40. Desroches, G. J., Gatenil, P. P., Nagao, K. & Macfarlane, R. J. Mechanical reinforcement of waterborne latex pressure‐sensitive adhesives with polymer‐grafted nanoparticles. J. Polym. Sci. (2023) doi:10.1002/pol.20230355.
41. Lewandowski et al. High Shear Pressure-Sensitive Adhesive. (2010).
Opening image courtesy of ipopba / iStock / Getty Images Plus.